Multiple Choice
Starting with cyclohexanone, how could you prepare the diketone below?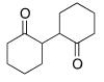
A) Treat cyclohexanone with a base under thermodynamic conditions.
B) Hydrogenate cyclohexanone with Raney nickel.
C) Convert cyclohexanone into the a-bromoketone and then react this with the enolate of cyclohexanone.
D) Convert cyclohexanone into an enamine with diethylamine and then react this with more cyclohexanone.
Correct Answer:

Verified
Correct Answer:
Verified
Q4: Why is it difficult to stop the
Q5: What are the three steps in the
Q7: Will acetophenone be completely deprotonated by lithium
Q19: What is the missing reagent in the
Q23: Vitamin C is a stable enediol.Which is
Q27: <sup> </sup>The reaction below is a direct
Q28: Which of the following four compounds is
Q29: <sub> </sub>The malonic ester synthesis can be
Q40: Which of the following bases will completely
Q41: Why is the enolate of acetone less