Multiple Choice
The reaction below is a direct enolate alkylation.It has been found that this reaction only works well with unhindered methyl and 1° alkyl halides.Pick the statement that best explains this observation. 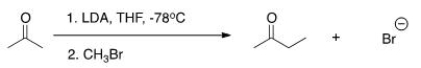
A) The nucleophilic enolate requires a reaction center that has a positive charge.
B) Hindered alkyl halides do not undergo SN1 reactions.
C) Hindered alkyl halides do not undergo SN2 reactions.
D) Methyl and 1° alkyl halides can form carbocations that can readily react with the nucleophilic enolate.
Correct Answer:

Verified
Correct Answer:
Verified
Q4: Why is it difficult to stop the
Q5: What are the three steps in the
Q7: Will acetophenone be completely deprotonated by lithium
Q23: Vitamin C is a stable enediol.Which is
Q24: Starting with cyclohexanone, how could you prepare
Q28: Which of the following four compounds is
Q29: <sub> </sub>The malonic ester synthesis can be
Q31: What is the starting material in the
Q32: The following molecule is called:<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5872/.jpg" alt="The
Q47: Will acetone be completely deprotonated by potassium