Multiple Choice
Why would the alcohol in the following compound need to be protected before reaction?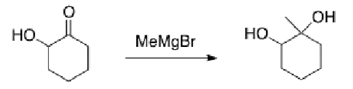
A) If it isn't protected, the product will be a carboxylic acid.
B) The Grignard reagent will react with the alcohol before the ketone.
C) Magnesium is Lewis acidic and will coordinate with the alcohol.
D) There is no need to protect the alcohol.
Correct Answer:

Verified
Correct Answer:
Verified
Q2: What will be the product of the
Q3: What reagent would be used to reduce
Q6: What is the product of the following
Q7: What is the starting material in the
Q8: What is the product of the following
Q10: Why are ketones less reactive than aldehydes?<br>A)Ketones
Q11: What is the missing reagent in the
Q26: What is the major organic product of
Q45: Which reagent can be used to reduce
Q57: Both LiAlH<sub>4</sub> and NaBH<sub>4</sub> are reducing agents.Which