Multiple Choice
The figure shows the electrolysis of molten NaI.What reaction occurs at the anode of this cell? 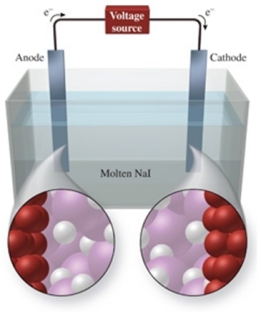
A) NaI(l) NaI2(l)
B) I-(l) + e- I2-
C) Na+(l) + e- Na(l)
D) 2I-(l) + e- I2(g)
E) 2I-(l) I2(g) + 2e-
Correct Answer:

Verified
Correct Answer:
Verified
Q3: Equations that are written to represent either
Q42: In which of the following choices is
Q70: The products of the electrolysis of molten
Q82: The following reaction occurs in a lead
Q97: The following reaction occurs in a lead
Q100: Consider the reaction: H<sub>2</sub>O(l) + 3SO<sub>3</sub><sup>2</sup><sup>-</sup>(aq)
Q103: The figure shows a molecular-level representation
Q104: Consider the half-reaction NO<sub>3</sub>-(aq) <span
Q105: What are the oxidation numbers of the
Q119: Sodium is produced by electrolysis of molten