Multiple Choice
From the vapor pressure curve of propane, it can be seen that the normal boiling point of propane is about _________. 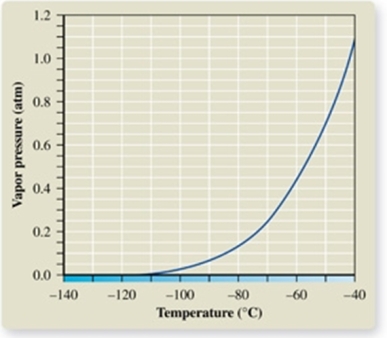
A) -137oC
B) -120oC
C) -60oC
D) -42oC
E) 0oC
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q27: Which of the following statements regarding intermolecular
Q30: Which of the following statements regarding the
Q32: Calculate the amount of heat energy
Q33: Of the two molecules shown in the
Q35: Which of the following substances is most
Q37: What phase change is occurring in the
Q39: Three phases of water are shown in
Q40: Rank the following substances in order of
Q46: Which choice correctly lists the intermolecular forces
Q95: Which of the following is not an