True/False
Of the two molecules shown in the figure, one would expect that CO2 has a higher boiling point than NO2 because CO2 is linear and nonpolar. 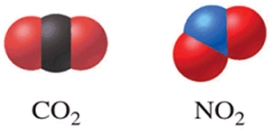
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q22: Which choice correctly lists the intermolecular forces
Q27: Which of the following statements regarding intermolecular
Q29: Which of the following has the strongest
Q30: Which of the following statements regarding the
Q32: Calculate the amount of heat energy
Q35: Which of the following substances is most
Q36: From the vapor pressure curve of propane,
Q37: What phase change is occurring in the
Q46: Which choice correctly lists the intermolecular forces
Q106: A solid substance has a very low