Multiple Choice
Which of the following is the best (simplest) balanced equation to represent the chemical reaction shown in the figure on any scale? 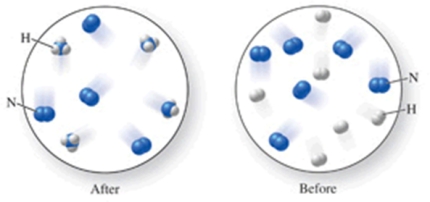
A) 12N + 12H 12NH
B) 6N2 + 6H2 4NH3
C) 6N2 + 6H2 4NH3 + 4N2
D) 12N + 12H 4NH3 + 8N
E) N2 + 3H2 2NH3
Correct Answer:

Verified
Correct Answer:
Verified
Q47: Phosphine, PH<sub>3</sub>, a reactive and poisonous
Q48: Nitrogen monoxide reacts with oxygen according
Q49: The following reaction absorbs 393 kJ
Q50: When mercury(II) oxide, a red crystalline
Q51: In the process of obtaining lead
Q53: Consider the reaction between acetylene, C<sub>2</sub>H<sub>2</sub>,
Q54: A 2.50 g sample of pitted
Q55: Nitrogen monoxide reacts with oxygen according
Q56: The figure shows a molecular-level diagram
Q57: Aluminum reacts with oxygen according to