Multiple Choice
The figure shows a molecular-level diagram of reactant molecules for the reaction: 2C2H2(g) + 5O2(g) 4CO2(g) + 2H2O(g) 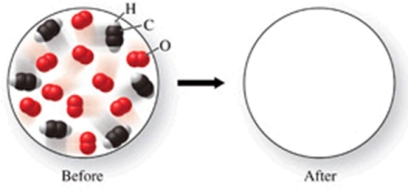 List the number and formulas of the molecules that should be present after the reaction takes place.
List the number and formulas of the molecules that should be present after the reaction takes place.
A) 4CO2 + 2H2O
B) 4CO2 + 2H2O + 2C2H2
C) 4CO2 + 2H2O + 2C2H2 + 5O2
D) 6CO2 + 3H2O + 3O2
E) 8CO2 + 4H2O + 2C2H2
Correct Answer:

Verified
Correct Answer:
Verified
Q51: In the process of obtaining lead
Q52: Which of the following is the
Q53: Consider the reaction between acetylene, C<sub>2</sub>H<sub>2</sub>,
Q54: A 2.50 g sample of pitted
Q55: Nitrogen monoxide reacts with oxygen according
Q57: Aluminum reacts with oxygen according to
Q58: What is the heat change when
Q59: Consider the following specific heats of metals.
Q60: Given the balanced equation 4NH<sub>3</sub>(g) +
Q61: How much heat energy would be