Multiple Choice
The figure shows a molecular-level diagram of reactant molecules for the reaction 2H2(g) + O2(g) 2H2O(l) 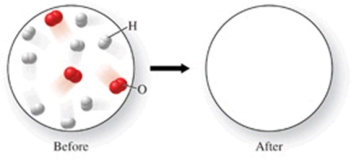 List the number and formulas of the molecules that should be present after the reaction takes place.
List the number and formulas of the molecules that should be present after the reaction takes place.
A) 2H2O + 6H2 + 2O2
B) 3H2O + 5H2 + O2
C) 4H2O + 4H2 + O2
D) 6H2O + 2H2 + O2
E) 6H2O + 2H2
Correct Answer:

Verified
Correct Answer:
Verified
Q32: Which of the following best describes an
Q42: A pamphlet requires one cover, 14 pieces
Q66: When 3.0 mol CaCl<sub>2</sub> dissolves in water,
Q71: When phosphorus reacts with chlorine, phosphorus
Q72: If 75.0 J of heat energy is
Q73: When a 0.525 g piece of
Q76: Phosphine, PH<sub>3</sub>, a reactive and poisonous
Q77: Ammonia is usually made by the
Q78: When mercury(II) oxide, a red crystalline
Q79: Given that 4NH<sub>3</sub>(g) + 5O<sub>2</sub>(g)