Multiple Choice
If 75.0 J of heat energy is added to 25.0 g samples of different metals.Given their specific heat values, rank the metals in order from least to greatest final temperature. 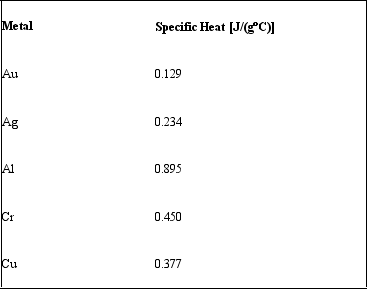
A) Au < Ag < Cu < Cr < Al
B) Al < Cr < Cu < Ag < Au
C) Au < Ag < Al < Cr < Cu
D) Cr < Cu < Al < Ag < Au
E) none of these-all final temperatures would be equal
Correct Answer:

Verified
Correct Answer:
Verified
Q32: Which of the following best describes an
Q42: A pamphlet requires one cover, 14 pieces
Q66: When 3.0 mol CaCl<sub>2</sub> dissolves in water,
Q67: Consider the following reaction: 3NO<sub>2</sub>(g) +
Q68: When mixed, solutions of aluminum nitrate,
Q71: When phosphorus reacts with chlorine, phosphorus
Q73: When a 0.525 g piece of
Q74: The figure shows a molecular-level diagram
Q76: Phosphine, PH<sub>3</sub>, a reactive and poisonous
Q77: Ammonia is usually made by the