Multiple Choice
Consider the following specific heats of metals. 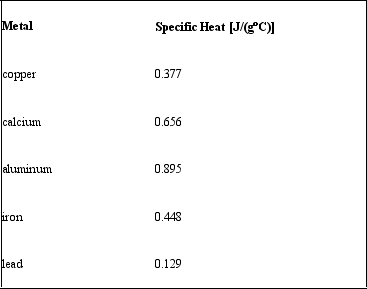 If the same amount of heat is added to 50.0 g of each of these metals, all at the same initial temperature, which metal will have the lowest final temperature?
If the same amount of heat is added to 50.0 g of each of these metals, all at the same initial temperature, which metal will have the lowest final temperature?
A) copper
B) calcium
C) aluminum
D) iron
E) lead
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q54: A 2.50 g sample of pitted
Q55: Nitrogen monoxide reacts with oxygen according
Q56: The figure shows a molecular-level diagram
Q57: Aluminum reacts with oxygen according to
Q58: What is the heat change when
Q60: Given the balanced equation 4NH<sub>3</sub>(g) +
Q61: How much heat energy would be
Q62: Consider the reaction between sodium metal
Q63: Aluminum reacts with oxygen according to
Q64: How much heat energy would be