Multiple Choice
The isotopes 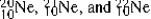 are all stable, while
are all stable, while 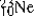 is radioactive. The mode of decay for
is radioactive. The mode of decay for 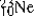 is most likely to be
is most likely to be
A) positron decay.
B) α decay.
C) γ decay.
D) electron capture.
E) β decay.
Correct Answer:

Verified
Correct Answer:
Verified
Q11: Exposure to 10 nCi for 10 minutes
Q12: The isotope <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8248/.jpg" alt="The isotope
Q13: Gamma rays are high energy electrons.
Q14: Calcium-39 undergoes positron decay. Each positron carries
Q15: The radioisotope <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8248/.jpg" alt="The radioisotope
Q17: Which of the following isotopes is most
Q18: The difference between the rad and the
Q19: A 9.52 × 10<sup>-</sup><sup>5</sup> mol sample of
Q20: Carbon-14 will emit a β particle with
Q21: Cesium-134 is a β emitter with a