Multiple Choice
Which of the following isotopes is most likely to be unstable?
A) 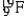
B) 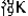
C) 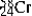
D) 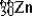
E) 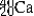
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q12: The isotope <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8248/.jpg" alt="The isotope
Q13: Gamma rays are high energy electrons.
Q14: Calcium-39 undergoes positron decay. Each positron carries
Q15: The radioisotope <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8248/.jpg" alt="The radioisotope
Q16: The isotopes <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8248/.jpg" alt="The isotopes
Q18: The difference between the rad and the
Q19: A 9.52 × 10<sup>-</sup><sup>5</sup> mol sample of
Q20: Carbon-14 will emit a β particle with
Q21: Cesium-134 is a β emitter with a
Q22: Palladium-107 undergoes β decay (t<sub>1/2</sub> = 6.5