Multiple Choice
Calculate ΔS° for the reaction 4Cr(s) + 3O2(g) → 2Cr2O3(s) 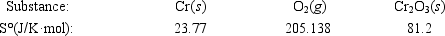
A) −548.1 J/K
B) −147.7 J/K
C) 147.7 J/K
D) 310.1 J/K
E) 548.1 J/K
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q65: The formation constant for the reaction Ag<sup>+</sup>(aq)
Q66: Which of the following is true for
Q67: Which of the following is necessary for
Q68: Which relationship or statement best describes ΔS°
Q69: Which of the following conditions will ensure
Q71: Calculate ΔS° for the combustion of propane.
Q72: A certain process has ΔH° > 0,
Q73: Which relationship or statement best describes ΔS°
Q74: Which of the following results in a
Q75: The second law of thermodynamics tells us