Multiple Choice
Calculate ΔS° for the combustion of propane. C3H8(g) + 5O2(g) → 3CO2(g) + 4H2O(g) 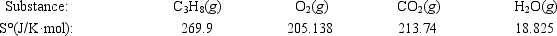
A) −100.9 J/K
B) −72.5 J/K
C) 72.5 J/K
D) 100.9 J/K
E) 877.5 J/K
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q66: Which of the following is true for
Q67: Which of the following is necessary for
Q68: Which relationship or statement best describes ΔS°
Q69: Which of the following conditions will ensure
Q70: Calculate ΔS° for the reaction 4Cr(s) +
Q72: A certain process has ΔH° > 0,
Q73: Which relationship or statement best describes ΔS°
Q74: Which of the following results in a
Q75: The second law of thermodynamics tells us
Q76: Calculate ΔS° for the reaction SiCl<sub>4</sub>(g) +