Multiple Choice
Elemental boron can be formed by reaction of boron trichloride with hydrogen. BCl3(g) + 1.5H2(g) → B(s) + 3HCl(g) 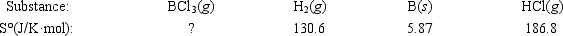 If ΔS° = 80.3 J/K for the reaction above, what is S° for BCl3(g) ?
If ΔS° = 80.3 J/K for the reaction above, what is S° for BCl3(g) ?
A) −18.2 J/K·mol
B) 18.2 J/K·mol
C) 290.1 J/K·mol
D) 355.4 J/K.mol
E) 450.6 J/K·mol
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q41: Which of the following is always true
Q42: For a process with ΔS < 0,
Q43: Calculate ΔG° for the reaction of ammonia
Q44: When a sky diver free-falls through the
Q45: Use the given data at 298 K
Q47: In a spontaneous process, the entropy of
Q48: Which of the following pairs has the
Q49: Which relationship best describes ΔS° for the
Q50: For a given reaction, a change in
Q51: Select the correct statement of a law