Multiple Choice
Use the given data at 298 K to calculate ΔG° for the reaction 2Cl2(g) + SO2(g) → SOCl2(g) + Cl2O(g) 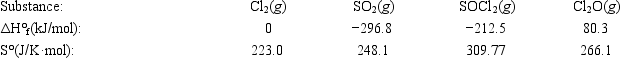
A) 129.3 kJ
B) 133.6 kJ
C) 196.0 kJ
D) 199.8 kJ
E) 229.6 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q40: For any reaction, if ΔG° > 0,
Q41: Which of the following is always true
Q42: For a process with ΔS < 0,
Q43: Calculate ΔG° for the reaction of ammonia
Q44: When a sky diver free-falls through the
Q46: Elemental boron can be formed by reaction
Q47: In a spontaneous process, the entropy of
Q48: Which of the following pairs has the
Q49: Which relationship best describes ΔS° for the
Q50: For a given reaction, a change in