Multiple Choice
Calculate ΔG° for the reaction SiCl4(g) + 2Mg(s) → 2MgCl2(s) + Si(s) 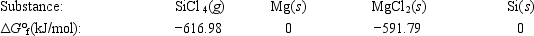
A) 566.60 kJ
B) 50.38 kJ
C) 25.19 kJ
D) −25.19 kJ
E) −566.60 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q75: The second law of thermodynamics tells us
Q76: Calculate ΔS° for the reaction SiCl<sub>4</sub>(g) +
Q77: Consider the figure that shows ΔG° for
Q78: You are given pure samples of ethane,
Q79: Calculate ΔG° for the combustion of propane.
Q80: Which relationship or statement best describes ΔS°
Q81: Which of the following is always true
Q82: You are given pure samples of pentane,
Q83: Which one of the following changes of
Q85: Iron(III) oxide can be reduced by carbon