Multiple Choice
Calculate ΔG° for the combustion of propane. C3H8(g) + 5O2(g) → 3CO2(g) + 4H2O(g) 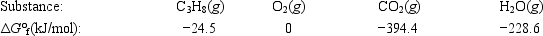
A) −2073.1 kJ
B) −1387.3 kJ
C) −598.5 kJ
D) 598.5 kJ
E) 2073.1 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q74: Which of the following results in a
Q75: The second law of thermodynamics tells us
Q76: Calculate ΔS° for the reaction SiCl<sub>4</sub>(g) +
Q77: Consider the figure that shows ΔG° for
Q78: You are given pure samples of ethane,
Q80: Which relationship or statement best describes ΔS°
Q81: Which of the following is always true
Q82: You are given pure samples of pentane,
Q83: Which one of the following changes of
Q84: Calculate ΔG° for the reaction SiCl<sub>4</sub>(g) +