Multiple Choice
The temperature at which the following process reaches equilibrium at 1.0 atm is the normal boiling point of hydrogen peroxide. H2O2(l) ⇄ H2O2(g)
Use the following thermodynamic information at 298 K to determine this temperature.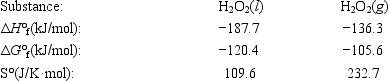
A) 120°C
B) 144°C
C) 196°C
D) 418°C
E) 585°C
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q47: In a spontaneous process, the entropy of
Q48: Which of the following pairs has the
Q49: Which relationship best describes ΔS° for the
Q50: For a given reaction, a change in
Q51: Select the correct statement of a law
Q53: The entropy of one mole of oxygen
Q54: In order for a process to be
Q55: In order for a process to be
Q56: The reaction of methane with water to
Q57: A certain process has ΔS<sub>univ</sub> > 0