Multiple Choice
The reaction of methane with water to form carbon dioxide and hydrogen is nonspontaneous at 298 K. At what temperature will this system make the transition from nonspontaneous to spontaneous? The data refer to 298 K. CH4(g) + 2H2O(g) ⇄ CO2(g) + 4H2(g) 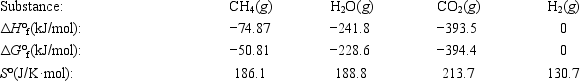
A) 658 K
B) 683 K
C) 955 K
D) 1047 K
E) 1229 K
Correct Answer:

Verified
Correct Answer:
Verified
Q51: Select the correct statement of a law
Q52: The temperature at which the following process
Q53: The entropy of one mole of oxygen
Q54: In order for a process to be
Q55: In order for a process to be
Q57: A certain process has ΔS<sub>univ</sub> > 0
Q58: In some spontaneous processes, the entropy of
Q59: Use the thermodynamic data at 298 K
Q60: Given: H<sub>2</sub>O(l) → H<sub>2</sub>O(g) ΔH° = 40.7
Q61: The temperature at which the following process