Multiple Choice
The temperature at which the following process reaches equilibrium at 1.0 atm is the normal melting point for phosphoric acid. H3PO4(s) ⇄ H3PO4(l)
Use the following thermodynamic information at 298 K to determine this temperature.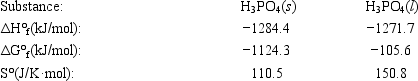
A) 286 K
B) 305 K
C) 315 K
D) 347 K
E) 3170 K
Correct Answer:

Verified
Correct Answer:
Verified
Q56: The reaction of methane with water to
Q57: A certain process has ΔS<sub>univ</sub> > 0
Q58: In some spontaneous processes, the entropy of
Q59: Use the thermodynamic data at 298 K
Q60: Given: H<sub>2</sub>O(l) → H<sub>2</sub>O(g) ΔH° = 40.7
Q62: Consider the figure that shows ΔG° for
Q63: Which, if any, of the following processes
Q64: For a chemical reaction to be spontaneous
Q65: The formation constant for the reaction Ag<sup>+</sup>(aq)
Q66: Which of the following is true for