Multiple Choice
Diethyl ether, used as a solvent for extraction of organic compounds from aqueous solutions, has a high vapor pressure which makes it a potential fire hazard in laboratories in which it is used. How much energy is released when 100.0 g is cooled from 53.0°C to 10.0°C? 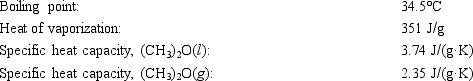
A) 10.1 kJ
B) 13.1 kJ
C) 16.1 kJ
D) 45.2 kJ
E) 48.6 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Q57: When liquid bromine is cooled to form
Q58: Which one of the following statements about
Q59: Select the pair of substances in which
Q60: The strongest intermolecular interactions between ethyl alcohol
Q61: What types of forces exist between molecules
Q63: The phase diagram of a substance shows
Q64: Which of the following should have the
Q65: Liquid crystal displays are most commonly constructed
Q66: Examine the phase diagram for the substance
Q67: The phase diagram for xenon has a