Multiple Choice
Examine the phase diagram for the substance Bogusium (Bo) and select the correct statement. 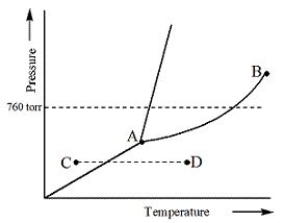
A) Bo( s) has a lower density than Bo( l) .
B) The triple point for Bo is at a higher temperature than the melting point for Bo.
C) Bo changes from a solid to a liquid as one follows the line from C to D.
D) Bo changes from a liquid to a gas as one follows the line from C to D.
E) Point B represents the critical temperature and pressure for Bo.
Correct Answer:

Verified
Correct Answer:
Verified
Q61: What types of forces exist between molecules
Q62: Diethyl ether, used as a solvent for
Q63: The phase diagram of a substance shows
Q64: Which of the following should have the
Q65: Liquid crystal displays are most commonly constructed
Q67: The phase diagram for xenon has a
Q68: Hexagonal close packing of identical atoms occurs
Q69: Examine the following phase diagram and identify
Q70: Examine the following phase diagram and identify
Q71: Select the pair of substances in which