Multiple Choice
Select the best Lewis structure for P2I4.
A) 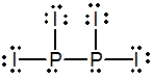
B) 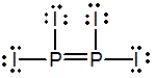
C) 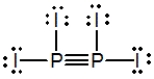
D) 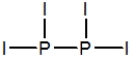
E) None of these choices are correct.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q3: In the following Lewis structure for ClO<sub>3</sub>F,
Q4: Select the Lewis structure for XeO<sub>2</sub>F<sub>2 </sub>that
Q5: The Lewis structure of NO<sub>2</sub> violates the
Q6: All possible resonance structures contribute equally to
Q7: Boron never achieves an octet in any
Q9: Which one of the following molecules contains
Q10: Predict the ideal bond angles in GeCl<sub>4
Q11: When resonance occurs, the bond lengths in
Q12: What is the molecular shape of NH<sub>2</sub>Cl
Q13: Which of the following molecules has a