Multiple Choice
In the following Lewis structure for ClO3F, chlorine has a formal charge of __________ and an oxidation number of __________. 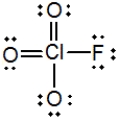
A) 7; 7
B) 7; −1
C) 1; 1
D) 1; −1
E) 1; 7
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: Which one of the following molecules has
Q2: Phosphoryl iodide is used in the preparation
Q4: Select the Lewis structure for XeO<sub>2</sub>F<sub>2 </sub>that
Q5: The Lewis structure of NO<sub>2</sub> violates the
Q6: All possible resonance structures contribute equally to
Q7: Boron never achieves an octet in any
Q8: Select the best Lewis structure for P<sub>2</sub>I<sub>4</sub>.<br>A)
Q9: Which one of the following molecules contains
Q10: Predict the ideal bond angles in GeCl<sub>4
Q11: When resonance occurs, the bond lengths in