Multiple Choice
The formal charge on Cl in the structure shown for the perchlorate ion is 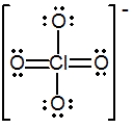
A) −2
B) −1
C) 0
D) +1
E) +2
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q44: Predict the ideal bond angles in AsCl<sub>3
Q45: Bond angles of 180° only occur around
Q46: What is the molecular shape of ClF<sub>4</sub><sup>−</sup>
Q47: Select the correct Lewis structure for TeBr<sub>2</sub>.<br>A)
Q48: Use VSEPR theory to decide which one
Q50: According to VSEPR theory, a molecule with
Q51: What is the molecular shape of ClF<sub>2</sub><sup>−</sup>
Q52: What is the molecular shape of XeO<sub>2</sub>F<sub>2
Q53: Which of the following atoms can expand
Q54: In neutral molecules, how many bonds are