Multiple Choice
What is the molecular shape of XeO2F2 as predicted by the VSEPR theory? 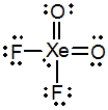
A) Square planar
B) Tetrahedral
C) Square pyramidal
D) See-saw
E) Octahedral
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q47: Select the correct Lewis structure for TeBr<sub>2</sub>.<br>A)
Q48: Use VSEPR theory to decide which one
Q49: The formal charge on Cl in the
Q50: According to VSEPR theory, a molecule with
Q51: What is the molecular shape of ClF<sub>2</sub><sup>−</sup>
Q53: Which of the following atoms can expand
Q54: In neutral molecules, how many bonds are
Q55: According to VSEPR theory, a molecule with
Q56: In which one of the following molecules
Q57: In formaldehyde, CH<sub>2</sub>O, both the formal charge