Multiple Choice
The figure shows the electrolysis of molten NaI. What reaction occurs at the anode of this cell? 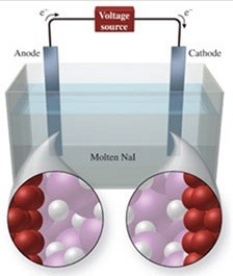
A) NaI(l) → NaI2(l)
B) I−(l) + e− → I2−
C) Na+(l) + e− → Na(l)
D) 2I−(l) + e− → I2(g)
E) 2I−(l) → I2(g) + 2e−
Correct Answer:

Verified
Correct Answer:
Verified
Q91: The figure shows the electrolysis of molten
Q92: Consider the following reaction: Mg(s)+ NiSO<sub>4</sub>(aq)→ MgSO<sub>4</sub>(aq)+
Q93: The ion shown has a charge of
Q94: A lead-acid battery that is used in
Q95: A species that causes a decrease in
Q97: Consider the half-reaction NH<sup>4</sup>+(aq)→ NO<sup>3</sup>-(aq). When the
Q98: If two metals are in contact with
Q99: Examine the following reaction: 5FeCl<sub>2</sub>(aq)+ KMnO<sub>4</sub>(aq)+ 8HCl(aq)-
Q100: Consider the half-reaction ClO<sup>−</sup>(aq)→ Cl<sup>−</sup>(aq). When the
Q101: Given the following information about the activity