Multiple Choice
The figure shows the electrolysis of molten NaI. What reaction occurs at the anode of this cell? 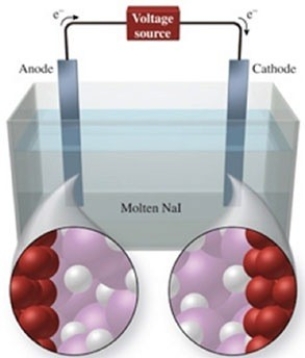
A) NaI(l) → Na+(l) + I−(l)
B) NaI(l) → Na(l) + I(g)
C) NaI(s) → Na+(aq) + I−(aq)
D) 2NaI(s) → 2Na(s) + I2(s)
E) 2NaI(l) → 2Na(l) + I2(g)
Correct Answer:

Verified
Correct Answer:
Verified
Q86: Batteries are electrochemical cells that are constructed
Q87: A lead-acid battery that is used in
Q88: In which substance does chlorine have an
Q89: Which of the following statements regarding oxidation-reduction
Q90: List the oxidation number of sulfur in
Q92: Consider the following reaction: Mg(s)+ NiSO<sub>4</sub>(aq)→ MgSO<sub>4</sub>(aq)+
Q93: The ion shown has a charge of
Q94: A lead-acid battery that is used in
Q95: A species that causes a decrease in
Q96: The figure shows the electrolysis of molten