Multiple Choice
Consider the reaction N2(g) + O2(g) → 2NO(g) . The molecular image represents a mixture of N2(g) and O2(g) just before reaction occurs. What is the limiting reactant, and how much of the excess reactant remains after the reaction is complete? The image contains 2 N2 molecules and 4 O2 molecules. 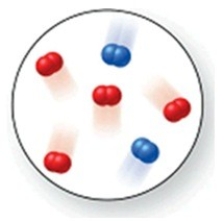
A) N2(g) , 1 O2(g)
B) N2(g) , 2 O2(g)
C) N2(g) , 3 O2(g)
D) O2(g) , 1 N2(g)
E) N2(g) , 0 O2(g)
Correct Answer:

Verified
Correct Answer:
Verified
Q87: A 2.50 g sample of pitted prunes
Q88: When mixed, solutions of silver nitrate, AgNO<sub>3</sub>,
Q89: The q value for the following reaction
Q90: When potassium metal is exposed to air,
Q91: Consider the following specific heats of metals.
Q93: When 5.0 g CaCl<sub>2</sub> is dissolved in
Q94: Given that 4NH<sub>3</sub>(g)+ 5O<sub>2</sub>(g)→ 4NO(g)+ 6H<sub>2</sub>O(g), if
Q95: Aluminum metal reacts with sulfuric acid according
Q96: Consider the following reaction: Cr<sub>2</sub>O<sub>3</sub>(s)+ 3CCl<sub>4</sub>(l)→ 2CrCl<sub>3</sub>(s)+
Q97: Which of the following processes is endothermic?<br>A)burning