Multiple Choice
Consider the following specific heats of metals. 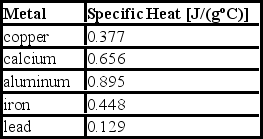 If the same amount of heat is added to 25.0 g of each of these metals, all at the same initial temperature, which metal will have the highest final temperature?
If the same amount of heat is added to 25.0 g of each of these metals, all at the same initial temperature, which metal will have the highest final temperature?
A) copper
B) calcium
C) aluminum
D) iron
E) lead
Correct Answer:

Verified
Correct Answer:
Verified
Q86: Phosphine, PH<sub>3</sub>, a reactive and poisonous compound,
Q87: A 2.50 g sample of pitted prunes
Q88: When mixed, solutions of silver nitrate, AgNO<sub>3</sub>,
Q89: The q value for the following reaction
Q90: When potassium metal is exposed to air,
Q92: Consider the reaction N<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2NO(g). The
Q93: When 5.0 g CaCl<sub>2</sub> is dissolved in
Q94: Given that 4NH<sub>3</sub>(g)+ 5O<sub>2</sub>(g)→ 4NO(g)+ 6H<sub>2</sub>O(g), if
Q95: Aluminum metal reacts with sulfuric acid according
Q96: Consider the following reaction: Cr<sub>2</sub>O<sub>3</sub>(s)+ 3CCl<sub>4</sub>(l)→ 2CrCl<sub>3</sub>(s)+