Multiple Choice
The relation PV = nRT holds for all ideal gases. The additional relation PVγ holds for an adiabatic process. The figure below shows two curves: one is an adiabat and one is an isotherm. Each starts at the same pressure and volume. Which statement is correct? (Note: "∝" means "is proportional to".) 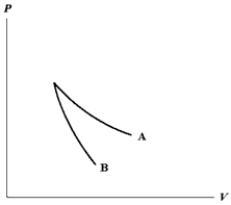
A) Isotherm: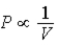 ; Adiabat:
; Adiabat: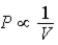 : A is both an isotherm and an adiabat.
: A is both an isotherm and an adiabat.
B) Isotherm: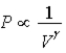 ; Adiabat:
; Adiabat: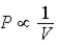 : B is an isotherm, A is an adiabat.
: B is an isotherm, A is an adiabat.
C) Isotherm: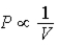 ; Adiabat:
; Adiabat: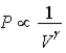 : A is an isotherm, B is an adiabat.
: A is an isotherm, B is an adiabat.
D) Isotherm: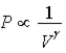 ; Adiabat:
; Adiabat: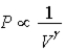 : B is both an isotherm and an adiabat.
: B is both an isotherm and an adiabat.
E) cannot answer without additional information about the starting temperature.
Correct Answer:

Verified
Correct Answer:
Verified
Q1: The temperature of a quantity of an
Q2: According to kinetic theory, a typical gas
Q3: When we consider a thin horizontal layer
Q5: During the volcanic eruption of Mt. Pelee
Q6: Nitrogen gas is heated by a pulsed
Q7: Air expands adiabatically (no heat in, no
Q8: When we say that the speed of
Q9: Find the specific heat (in cal/mole K)
Q10: The molar specific heat at constant volume
Q11: One mole of hydrogen, one mole of