Multiple Choice
Molecular speeds: If we double the root-mean-square speed (thermal speed) of the molecules of a gas, then
A) its temperature must increase by a factor of 4.
B) its temperature must increase by a factor of 2.
C) its temperature must increase by a factor of 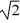 .
.
D) its pressure must increase by a factor of 2.
E) its pressure must increase by a factor of 4.
Correct Answer:

Verified
Correct Answer:
Verified
Q31: Molecular speeds: If the temperature of a
Q32: Radiation: Betelgeuse is a red supergiant star
Q33: Mean free path: An ideal gas is
Q34: Phase changes: A substance has a melting
Q35: Quantity of heat: A thermally isolated system
Q37: Thermal expansion: The coefficient of volume expansion
Q38: Ideal gas law: A bag of potato
Q39: Ideal gas law: A 25-L container holds
Q40: Conduction of heat: A cylindrical bar that
Q41: Radiation: What is the net power that