Multiple Choice
Phase changes: A substance has a melting point of 20°C and a heat of fusion of 3.9 × 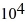 J/kg. The boiling point is
J/kg. The boiling point is 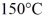 and the heat of vaporization is
and the heat of vaporization is 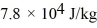 at a pressure of 1.0 atm. The specific heats for the solid, liquid, and gaseous phases are 600 J/(kg ∙ K) , 1000 J/(kg∙K) , and 400 J/(kg ∙ K) , respectively. The quantity of heat required to raise the temperature of
at a pressure of 1.0 atm. The specific heats for the solid, liquid, and gaseous phases are 600 J/(kg ∙ K) , 1000 J/(kg∙K) , and 400 J/(kg ∙ K) , respectively. The quantity of heat required to raise the temperature of 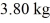 of the substance from
of the substance from 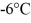 to
to 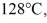 at a pressure of 1.0 atm, is closest to
at a pressure of 1.0 atm, is closest to
A) 620 kJ.
B) 470 kJ.
C) 560 kJ.
D) 210 kJ.
E) 770 kJ.
Correct Answer:

Verified
Correct Answer:
Verified
Q29: Calorimetry: A block of ice at 0.000°C
Q30: Conduction of heat: A concrete wall of
Q31: Molecular speeds: If the temperature of a
Q32: Radiation: Betelgeuse is a red supergiant star
Q33: Mean free path: An ideal gas is
Q35: Quantity of heat: A thermally isolated system
Q36: Molecular speeds: If we double the root-mean-square
Q37: Thermal expansion: The coefficient of volume expansion
Q38: Ideal gas law: A bag of potato
Q39: Ideal gas law: A 25-L container holds