Multiple Choice
Phase changes: Heat is added to a pure substance in a closed container at a constant rate. The figure shows a graph of the temperature of the substance as a function of time. If Lf = latent heat of fusion and Lv = latent heat of vaporization, what is the value of the ratio Lv / Lf for this substance? 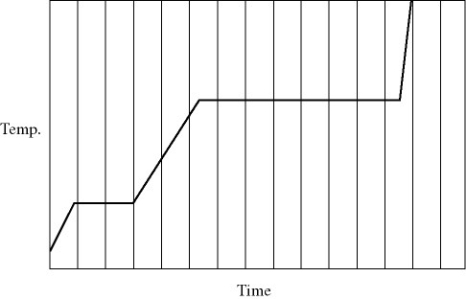
A) 5.0
B) 4.5
C) 7.2
D) 3.5
E) 1.5
Correct Answer:

Verified
Correct Answer:
Verified
Q104: Thermal expansion: Suppose that a steel bridge,
Q105: Molecular speeds: A container is filled with
Q106: Ideal gas law: A vertical tube that
Q107: Ideal gas law: 2.0 L of an
Q108: Conduction of heat: An architect is interested
Q110: Thermal expansion: Two steel spheres are made
Q111: Ideal gas law: The interior of a
Q112: Calorimetry: A copper cylinder with a mass
Q113: Calorimetry: A 400-g piece of metal at
Q114: Conduction of heat: The walls of an