Essay
Ideal gas law: A vertical tube that is closed at the upper end and open at the lower end contains an air pocket. The open end of the tube is under the water of a lake, as shown in the figure. When the lower end of the tube is just under the surface of the lake, where the temperature is 37°C and the pressure is 1.0 × 105 Pa, the air pocket occupies a volume of 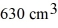 . Suppose now that the lower end of the tube is at a depth of 86 m in the lake, where the temperature is 7.0°C. What is the volume of the air pocket under these conditions? The density of the water in the lake is 1000 kg/m3.
. Suppose now that the lower end of the tube is at a depth of 86 m in the lake, where the temperature is 7.0°C. What is the volume of the air pocket under these conditions? The density of the water in the lake is 1000 kg/m3. 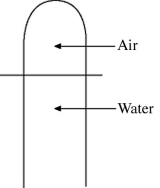
Correct Answer:

Verified
Correct Answer:
Verified
Q101: Ideal gas law: A hot air balloon
Q102: Thermal expansion: A rod has a length
Q103: Conduction of heat: A solid concrete wall
Q104: Thermal expansion: Suppose that a steel bridge,
Q105: Molecular speeds: A container is filled with
Q107: Ideal gas law: 2.0 L of an
Q108: Conduction of heat: An architect is interested
Q109: Phase changes: Heat is added to a
Q110: Thermal expansion: Two steel spheres are made
Q111: Ideal gas law: The interior of a