Multiple Choice
Phase changes: If you add 700 kJ of heat to 700 g of water at 70.0°C, how much water is left in the container? The latent heat of vaporization of water is 2.26 × 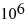 J/kg and its specific heat is 4190 J/(kg ∙ K) .
J/kg and its specific heat is 4190 J/(kg ∙ K) .
A) 429 g
B) 258 g
C) 340 g
D) 600 g
E) none
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q19: Molecular speeds: What is the average translational
Q20: Ideal gas law: An ideal gas is
Q21: Ideal gas law: Which contains more moles
Q22: Quantity of heat: It is necessary to
Q23: Phase changes: A chunk of ice (T
Q25: Mean free path: Assuming the radius of
Q26: Thermal expansion: The hole for a bolt
Q27: Ideal gas law: A cold trap is
Q28: Phase changes: A 406.0 kg copper bar
Q29: Calorimetry: A block of ice at 0.000°C