Multiple Choice
Mean free path: Assuming the radius of diatomic molecules is approximately 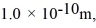 for what pressure will the mean free path in room-temperature (20°C) nitrogen be 4.6 m? The Boltzmann constant is 1.38 × 10-23 J/K, Avogadro's number is 6.02 × 1023 molecules/mole, and the ideal gas constant is R = 8.314 J/mol ∙ K = 0.0821 L ∙ atm/mol ∙ K.
for what pressure will the mean free path in room-temperature (20°C) nitrogen be 4.6 m? The Boltzmann constant is 1.38 × 10-23 J/K, Avogadro's number is 6.02 × 1023 molecules/mole, and the ideal gas constant is R = 8.314 J/mol ∙ K = 0.0821 L ∙ atm/mol ∙ K.
A) 4.9 × 10-8 atm
B) 6.9 × 10-8 atm
C) 1.5 × 10-7 atm
D) 2.2 × 10-7 atm
Correct Answer:

Verified
Correct Answer:
Verified
Q20: Ideal gas law: An ideal gas is
Q21: Ideal gas law: Which contains more moles
Q22: Quantity of heat: It is necessary to
Q23: Phase changes: A chunk of ice (T
Q24: Phase changes: If you add 700 kJ
Q26: Thermal expansion: The hole for a bolt
Q27: Ideal gas law: A cold trap is
Q28: Phase changes: A 406.0 kg copper bar
Q29: Calorimetry: A block of ice at 0.000°C
Q30: Conduction of heat: A concrete wall of