Multiple Choice
Phase changes: Heat is added to a 2.0 kg piece of ice at a rate of 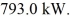 How long will it take for the ice to melt if it was initially at 0.00°C? (The latent heat of fusion for water is 334 kJ/kg and its latent heat of vaporization is 2260 kJ/kg.)
How long will it take for the ice to melt if it was initially at 0.00°C? (The latent heat of fusion for water is 334 kJ/kg and its latent heat of vaporization is 2260 kJ/kg.)
A) 0.84 s
B) 530,000 s
C) 4.7 s
D) 670 s
Correct Answer:

Verified
Correct Answer:
Verified
Q66: Ideal gas law: If a certain sample
Q67: Phase changes: If 2.0 g of water
Q68: Phase changes: When a solid melts,<br>A) the
Q69: Thermal expansion: A machinist needs to remove
Q70: Ideal gas law: A sealed 89- <img
Q72: Quantity of heat: A 905-g meteor impacts
Q73: Thermal expansion: The exterior of a supersonic
Q74: Molecular speeds: A 5.0-liter gas tank holds
Q75: Conduction of heat: What is the steady
Q76: Thermal expansion: A glass flask has a