Short Answer
Ideal gas law: If a certain sample of an ideal gas has a temperature of 109°C and exerts a pressure of 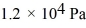 on the walls of its container, how many gas molecules are present in each cubic centimeter of volume? The ideal gas constant is 8.314 J/mol · K and Avogadro's number is 6.022 × 1023 molecules/mol.
on the walls of its container, how many gas molecules are present in each cubic centimeter of volume? The ideal gas constant is 8.314 J/mol · K and Avogadro's number is 6.022 × 1023 molecules/mol.
Correct Answer:

Verified
Correct Answer:
Verified
Q61: Conduction of heat: Two metal rods, one
Q62: Conduction of heat: A heat conducting rod,
Q63: Ideal gas law: A weather balloon contains
Q64: Mean free path: The mean free path
Q65: Calorimetry: A person pours 330 g of
Q67: Phase changes: If 2.0 g of water
Q68: Phase changes: When a solid melts,<br>A) the
Q69: Thermal expansion: A machinist needs to remove
Q70: Ideal gas law: A sealed 89- <img
Q71: Phase changes: Heat is added to a