Multiple Choice
Molar heat capacities: An expansion process on an ideal diatomic gas has a linear path between the initial and final states on a pV diagram. The initial pressure is 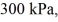 the initial volume is
the initial volume is 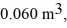 and the initial temperature is
and the initial temperature is 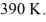 The ideal gas constant is R = 8.314 J/mol ∙ K. The final pressure is
The ideal gas constant is R = 8.314 J/mol ∙ K. The final pressure is 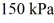 and the final temperature is
and the final temperature is 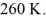 The work done by the gas is closest to
The work done by the gas is closest to
A) 4500 J.
B) 2300 J.
C) 3400 J.
D) 5600 J.
E) 6800 J.
Correct Answer:

Verified
Correct Answer:
Verified
Q41: First law of thermodynamics: An ideal gas
Q42: Molar heat capacities: 3.0 moles of an
Q43: First law of thermodynamics: A container of
Q44: Molar heat capacities: An adiabatic compression is
Q45: Type of thermodynamic processes: The figure shows
Q47: First law of thermodynamics: A cylinder contains
Q48: First law of thermodynamics: During an isothermal
Q49: First law of thermodynamics: In an isochoric
Q50: First law of thermodynamics: A cylinder contains
Q51: The figure shows the pV diagram for