Short Answer
The figure shows the pV diagram for a certain thermodynamic process. In this process, 1500 J of heat flows into a system, and at the same time the system expands against a constant external pressure of 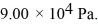 If the volume of the system increases from
If the volume of the system increases from 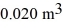 to
to 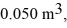 calculate the change in internal (thermal) energy of the system. If the internal (thermal) energy change is nonzero, be sure to indicate whether this energy change is positive or negative.
calculate the change in internal (thermal) energy of the system. If the internal (thermal) energy change is nonzero, be sure to indicate whether this energy change is positive or negative. 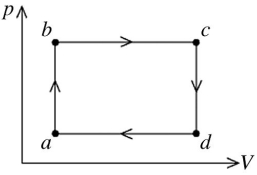
Correct Answer:

Verified
Correct Answer:
Verified
Q42: Molar heat capacities: 3.0 moles of an
Q43: First law of thermodynamics: A container of
Q44: Molar heat capacities: An adiabatic compression is
Q45: Type of thermodynamic processes: The figure shows
Q46: Molar heat capacities: An expansion process on
Q47: First law of thermodynamics: A cylinder contains
Q48: First law of thermodynamics: During an isothermal
Q49: First law of thermodynamics: In an isochoric
Q50: First law of thermodynamics: A cylinder contains
Q52: Work: An ideal gas in a balloon