Multiple Choice
What is the effect of adding CH3COOH(g) to a container in which the reaction has reached equilibrium? 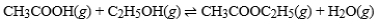
A) The reaction will shift in the forward direction.
B) The reaction will shift in the reverse direction.
C) There will be no effect.
D) The reaction will reach quasi-equilibrium.
Correct Answer:

Verified
Correct Answer:
Verified
Q64: Which of the following is true of
Q65: A particular chemical reaction carried out at
Q66: If the endothermic reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8310/.jpg" alt="If
Q67: For which of the following reactions is
Q68: Which of the following is true of
Q70: Suppose that the equilibrium constant for the
Q71: In a certain chemical reaction, 1.00 g
Q72: For the reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8310/.jpg" alt="For the
Q73: A catalyst speeds up a chemical reaction
Q74: Which of the following is not true