Multiple Choice
If the endothermic reaction 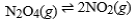 has reached equilibrium, what is the effect of raising the temperature of the reaction vessel?
has reached equilibrium, what is the effect of raising the temperature of the reaction vessel?
A) The reaction will shift from the left to the right.
B) The reaction will shift from the right to the left.
C) There will be no further reaction.
D) Whether the reaction will shift to the left or the right depends on the initial temperature.
Correct Answer:

Verified
Correct Answer:
Verified
Q61: Which of the following must we know
Q62: In an energy diagram for a chemical
Q63: Consider the following energy diagram for a
Q64: Which of the following is true of
Q65: A particular chemical reaction carried out at
Q67: For which of the following reactions is
Q68: Which of the following is true of
Q69: What is the effect of adding CH<sub>3</sub>COOH(g)
Q70: Suppose that the equilibrium constant for the
Q71: In a certain chemical reaction, 1.00 g