Multiple Choice
Consider the following graph. 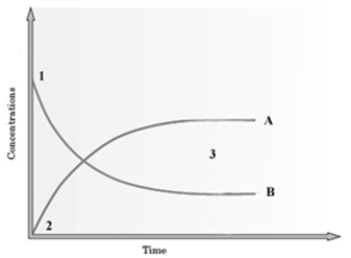 The graph is based on data collected from the following reaction.
The graph is based on data collected from the following reaction. 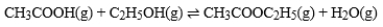 If Curve B presently represents C2H5OH, how would the graph change if this line represented CH3COOH?
If Curve B presently represents C2H5OH, how would the graph change if this line represented CH3COOH?
A) The slope of the line would greater.
B) The slope of the line would be less.
C) The line would be the same.
D) This condition cannot be predicted.
Correct Answer:

Verified
Correct Answer:
Verified
Q41: If a particular reactant is involved in
Q42: For the exothermic reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8310/.jpg" alt="For
Q43: The rate of reaction for the decomposition
Q44: What is the effect of decreasing the
Q45: In a particular chemical reaction, two bonds
Q47: If a reaction occurs very rapidly even
Q48: Given that the reaction 2Na<sub>2</sub>O (s) →
Q49: For the reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8310/.jpg" alt="For the
Q50: How does a decrease in temperature affect
Q51: Consider a reaction such as A<sub>2</sub>(g) +