Multiple Choice
For the exothermic reaction 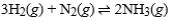 making which of the following changes will ensure that the reaction will not shift either to the left or to the right?
making which of the following changes will ensure that the reaction will not shift either to the left or to the right?
A) raising the temperature and adding NH3
B) lowering the temperature and removing NH3
C) raising the temperature and adding NH3 and lowering the temperature and removing NH3
D) none of these
Correct Answer:

Verified
Correct Answer:
Verified
Q37: Which of the following is true of
Q38: Consider the following graph. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8310/.jpg" alt="Consider
Q39: A particular reaction has an equilibrium constant
Q40: What is the purpose of the enteric
Q41: If a particular reactant is involved in
Q43: The rate of reaction for the decomposition
Q44: What is the effect of decreasing the
Q45: In a particular chemical reaction, two bonds
Q46: Consider the following graph. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8310/.jpg" alt="Consider
Q47: If a reaction occurs very rapidly even