Multiple Choice
Using the VSEPR model, predict which species have bond angles of about 109°.
HINT: Assume that the charges are correct. Add the missing lone pairs before applying the VSEPR theory!
A) I, III, IV 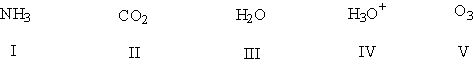
B) II, III, V
C) I, IV
D) III, IV, V
Correct Answer:

Verified
Correct Answer:
Verified
Q104: Which statement about contributing structures is false?<br>A)
Q105: Which of the three molecules aspirin, paracetamol
Q106: Using the VSEPR model, predict which molecules
Q107: The following carbocations are listed in increasing
Q108: The hydroboration and subsequent oxidation of 1-heptene
Q109: But-1-yn-1-ylcyclohexane reacts with molecular hydrogen under pressure
Q110: Which species are electrophiles? <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8498/.jpg" alt="Which
Q111: According to VSEPR model, what is your
Q112: Which one represents the activation energy? <img
Q113: The following molecule contains the _ and