Multiple Choice
What is E°cell for the following reaction? 2Fe2+(aq) + Cd2+(aq) → 2Fe3+(aq) + Cd(s) 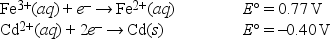
A) -0.37 V
B) 0.37 V
C) -1.17 V
D) 1.17 V
E) None of these choices is correct
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q64: Consider the following balanced redox reaction 3CuO(s)
Q91: Which equation is correct?<br>A) E = -(RT/nF)
Q92: Based on the data presented below, which
Q93: Based on the following electrochemical cell, which
Q94: What is the equilibrium constant at 25°C
Q95: Given the following standard reduction potentials, <img
Q97: What is meant by SHE?<br>A) Shared half
Q98: E > 0 and ΔG < 0
Q100: Which equation is correct?<br>A) E°<sub>cell</sub> = E°<sub>anode</sub>
Q101: Which metals may be oxidized by H<sup>+</sup>