Multiple Choice
Based on the following electrochemical cell, which statement is true? 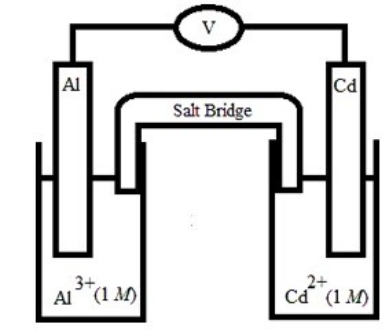
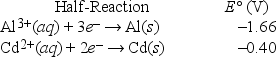
A) Al(s) is oxidized and is the anode.
B) Al(s) is oxidized and is the cathode.
C) Cd(s) is oxidized and is the anode.
D) Cd(s) is oxidized and is the cathode.
E) No reaction occurs because E°cell < 0.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q88: _ occurs at the cathode in a
Q89: What is the half-reaction that occurs at
Q90: A SHE has the acid concentration of
Q91: Which equation is correct?<br>A) E = -(RT/nF)
Q92: Based on the data presented below, which
Q94: What is the equilibrium constant at 25°C
Q95: Given the following standard reduction potentials, <img
Q96: What is E°<sub>cell</sub> for the following reaction?
Q97: What is meant by SHE?<br>A) Shared half
Q98: E > 0 and ΔG < 0